WHAT IS A BATTERY?
Introduction
Batteries are a collection of one or more cells whose chemical reactions create a flow of electrons in a circuit. All batteries are made up of three basic components: an anode (the ‘-’ side), a cathode (the ‘+’ side), and some kind of electrolyte (a substance that chemically reacts with the anode and cathode).
When the anode and cathode of a battery is connected to a circuit, a chemical reaction takes place between the anode and the electrolyte. This reaction causes electrons to flow through the circuit and back into the cathode where another chemical reaction takes place. When the material in the cathode or anode is consumed or no longer able to be used in the reaction, the battery is unable to produce electricity. At that point, your battery is “dead.”
Batteries that must be thrown away after use are known as primary batteries. Batteries that can be recharged are called secondary batteries.

Lithium polymer batteries, for example, can be recharged
Without batteries, your quadcopter would have to be tethered to the wall, you would have to hand crank your car, and your Xbox controller would have to plugged in all the time (like in the good old days). Batteries offer a way to store electrical potential energy in a portable container.

Batteries come in a variety of shapes, sizes, and chemistries.
The invention of the modern battery is often attributed to Alessandro Volta. It actually started with a surprising accident involving the dissection of a frog.
What You Will Learn
The following topics will be covered in detail in this tutorial:
How batteries were invented
What parts make up a battery
How a battery works
Common terms used to describe batteries
Various ways to use batteries in circuits
Suggested Reading
There are a few concepts that you might want to be familiar with before starting to read this guide:
History
Historically, the word “battery” was used to describe a “series of similar objects grouped together to perform a function,” as in a battery of artillery. In 1749, Benjamin Franklin first used the term to describe a series of capacitors he had linked together for his electricity experiments. Later, the term would be used for any electrochemical cells linked together for the purpose of providing electric power.

Battery of Leyden Jar “capacitors” linked together
(Image courtesy of Alvinrune of Wikimedia Commons)
Invention of the Battery
One fateful day in 1780, Italian physicist, physician, biologist, and philosopher, Luigi Galvani, was dissecting a frog attached to a brass hook. As he touched the frog’s leg with an iron scapel, the leg twitched. Galvani theorized that the energy came from the leg itself, but his fellow scientist, Alessandro Volta, believed otherwise.
Volta hypothesized that the frog’s leg impulses were actually caused by different metals soaked in a liquid. He repeated the experiment using cloth soaked in brine instead of a frog corpse, which resulted in a similar voltage. Volta published his findings in 1791 and later created the first battery, the voltaic pile, in 1800.
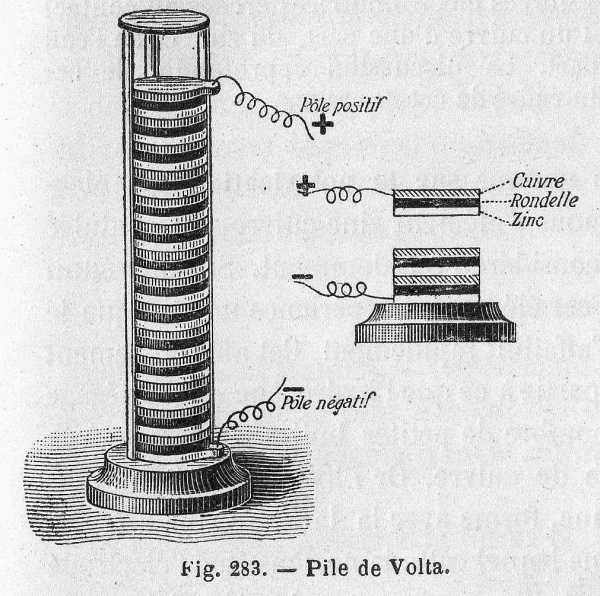
The voltaic pile consisted of a stack of zinc and copper plates separated by cloth soaked in brine
Volta’s pile was plagued by two major issues: the weight of the stack caused the electrolyte to leak out of the cloth, and the particular chemical properties of the components resulted in a very short life span (about an hour). The next two hundred years would be spent perfecting Volta’s design and solving these issues.
Fixes to the Voltaic Pile
William Cruickshank of Scotland solved the leakage problem by laying the voltaic pile on its side to form the “trough battery.”
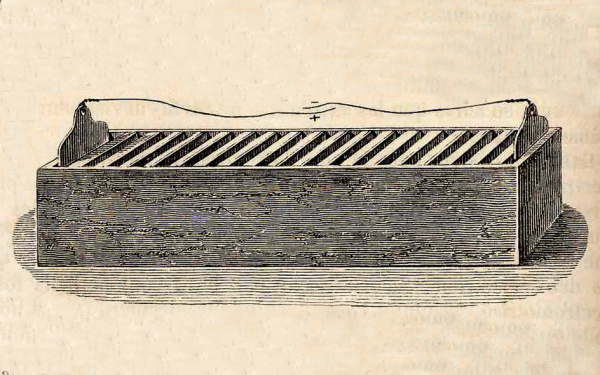
The trough battery solved the leakage problem of the voltaic pile
The second problem, short life span, was caused by the degradation of the zinc due to impurities and a build up of hydrogen bubbles on the copper. In 1835, William Sturgeon discovered that treating the zinc with mercury would prevent degradation.
The British chemist John Frederic Daniell used a second electrolyte that reacted with the hydrogen, preventing buildup on the copper cathode. Daniell’s two-electrolyte battery, known as the “Daniell cell,” would become a very popular solution to providing power to the budding telegraph networks.

A collection of Daniell cells from 1836
The First Rechargeable Battery
In 1859, the French physicist Gaston Planté created a battery using two rolled sheets of lead submerged in sulfuric acid. By reversing the electrical current through the battery, the chemistry would return to its original state, thus creating the first rechargeable battery.
Later, in 1881, Camille Alphonse Faure improved Planté’s design by forming the lead sheets into plates. This new design made the batteries easier to manufacture, and the lead acid battery saw wide-spread use in automobiles.

The design for the common “car battery” has been around for more than 100 years
(Image courtesy of Emilian Robert Vicol of Wikimedia Commons)
The Dry Cell
Up until the late 1800s, the electrolyte in batteries was in a liquid state. This made battery transportation a very careful endeavor, and most batteries were never intended to be moved once attached to the circuit.
In 1866, Georges Leclanché created a battery using a zinc anode, a manganese dioxide cathode, and an ammonium chloride solution for the electrolyte. While the electrolyte in the Leclanché cell was still a liquid, the battery’s chemistry proved to be an important step for the invention of the dry cell.
Carl Gassner figured out how to create an electrolyte paste out of ammonium chloride and Plaster of Paris. He patented the new “dry cell” battery in 1886 in Germany.
These new dry cells, commonly called “zinc-carbon batteries,” were massed produced and proved hugely popular until the late 1950s. While carbon is not used in the chemical reaction, it performs an important role as an electrical conductor in the zinc-carbon battery.

3V zinc-carbon battery from the 1960s
(Image courtesy of PhFabre of Wikimedia Commons)
In the 1950s, Lewis Urry, Paul Marsal, and Karl Kordesch of the Union Carbide company (later known as “Eveready” and then “Energizer”) replaced the ammonium chloride electrolyte with an alkaline substance, based on the battery chemistry formulated by Waldemar Jungner in 1899. Alkaline dry cell batteries could hold more energy than zinc carbon batteries of the same size and had a longer shelf life.
Alkaline batteries rose in popularity in the 1960s, overtook zinc-carbon batteries, and have since become the standard primary cell for consumer use.

Alkaline batteries come in many shapes and sizes
(Image courtesy of Aney~commonswiki of Wikimedia Commons)
20th Century Rechargeable Batteries
In the 1970s, COMSAT developed the nickel-hydrogen battery for use in communication satellites. These batteries store hydrogen in a pressurized, gaseous form. Many man-made satellites, like the International Space Station, still rely on nickel-hydrogen batteries.
The research of several companies since the late 1960s resulted in the createion of the nickel-metal hydride (NiMH) battery. NiMH batteries were released to the consumer market in 1989, and provided a smaller, cheaper alternative to the rechargeable nickel-hydrogen cells.
Asahi Chemical of Japan built the first lithium-ion battery in 1985, and Sony created the first commercial lithium-ion battery in 1991. In the late 1990s, a soft, flexible casing was created for lithium-ion batteries and gave rise to the “lithium polymer” or “LiPo” battery.
The chemical reactions in the lithium polymer battery are essentially the same as those in the lithium-ion battery
Obviously, many more battery chemistries have been invented, manufactured, and become obsolete. If you would like to read more about modern, popular battery technologies, check out our Battery Technologies tutorial.
Components
Batteries are made up of three basic components: an anode, a cathode, and an electrolyte. A separator is often used to prevent the anode and cathode from touching, if the electrolyte is not sufficient. In order to store these components, batteries usually have some kind of casing.
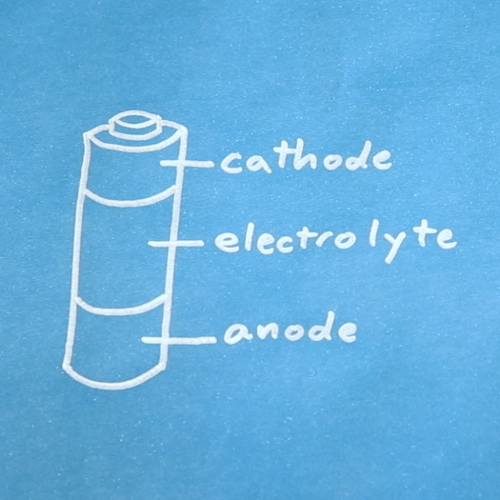
OK, most batteries are not actually divided up in three equal sections, but you get the idea. A better cross-section of an alkaline cell can be found on Wikipedia.
Both the anode and cathode are types of electrodes. Electrodes are conductors through which electricity enters or leaves a component in a circuit.
Anode
Electrons flow out from the anode in a device connected to a circuit. This means that conventional “current” flows into an anode.

On batteries, the anode is marked as the negative (-) terminal
In a battery, the chemical reaction between the anode and electrolyte causes a build up of electrons in the anode. These electrons want to move to the cathode, but cannot pass through the electrolyte or separator.
Cathode
Electrons flow into the cathode in a device connected to a circuit. This means that conventional “current” flows out from a cathode.

On batteries, the cathode is marked as the positive (+) terminal
In batteries, the chemical reaction in or around the cathode uses the electrons produced in the anode. The only way for the electrons to get to the cathode is through a circuit, external to the battery.
Electrolyte
The electrolyte is the substance, often a liquid or gel, that is capable of transporting ions between the chemical reactions that happen at the anode and cathode. The electrolyte also inhibits the flow of electrons between the anode and cathode so that the electrons more easily flow through the external circuit rather than through the electrolyte.

Alkaline batteries can leak their electrolyte, potassium hydroxide, if subjected to high heat or reverse voltage
(Image courtesy of Wiliam Davies of Wikimedia Commons)
The electrolyte is crucial in the operation of a battery. Because electrons cannot pass through it, they are forced to travel through electrical conductors in the form of a circuit that connect the anode to the cathode.
Separator
Separators are porous materials that prevent the anode and cathode from touching, which would cause a short circuit in the battery. Separators can be made from a variety of materials, including cotton, nylon, polyester, cardboard, and synthetic polymer films. Separators do not chemically react with either the anode, cathode, or electrolyte.
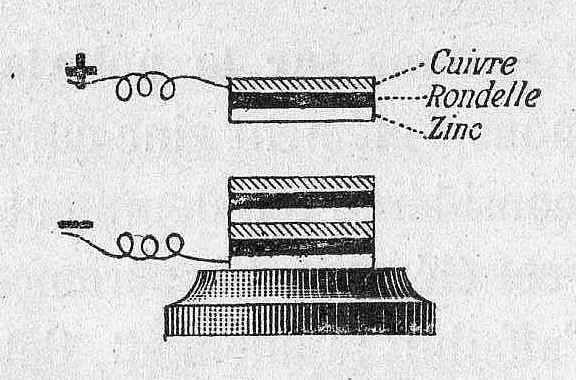
The voltaic pile used cloth or cardboard (separator) soaked in brine (electrolyte) to keep the electrodes apart
Ions in the electrolyte can be positively charged, negatively charged, and can come in a variety of sizes. Special separators can be manufactured that allow some ions to pass but not others.
Casing
Most batteries need a way to contain their chemical components. Casings, otherwise known as “housings” or “shells,” are simply mechanical structures meant to hold the battery’s internals.

This lead-acid battery has a plastic casing
Battery casings can be made of almost anything: plastic, steel, soft polymer laminate pouches, and so on. Some batteries use a conducting steel casing that is electrically connected to one of the electrodes. In the case of the common AA alkaline cell, the steel casing is connected to the cathode.
Operation
Batteries generally require several chemical reactions in order to operate. At least one reaction occurs in or around the anode and one or more reactions occur in or around the cathode. In all cases, the reaction at the anode produces extra electrons in a process called oxidation, and the reaction at the cathode uses the extra electrons during a process known as reduction.
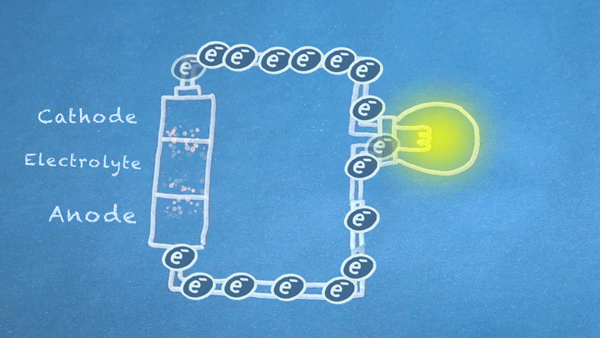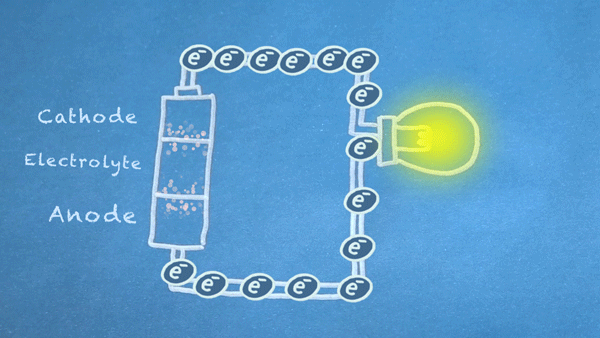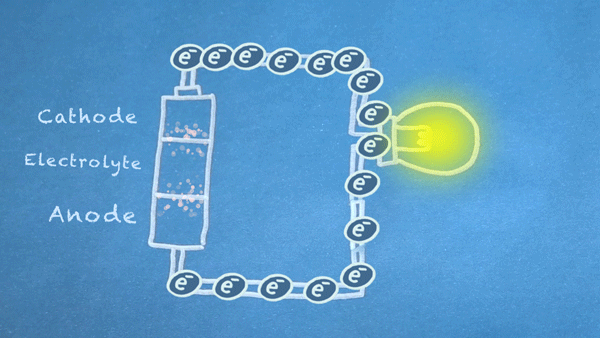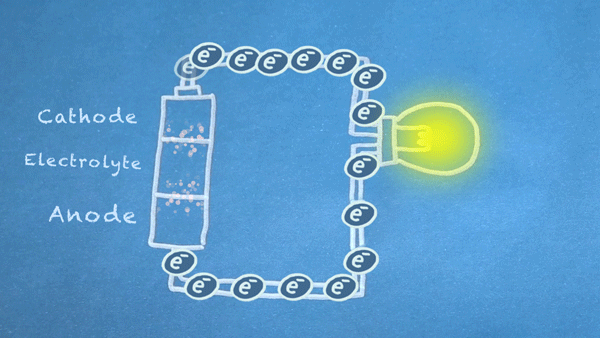
When the switch is closed, the circuit is complete, and electrons can flow from the anode to the cathode. These electrons enable the chemical reations at the anode and cathode.
In essence, we are separating a certain kind of chemical reaction, a reduction-oxidation reaction or redox reaction, into two separate parts. Redox reactions occur when electrons are transferred between chemicals. We can harness the movement of electrons in this reaction to flow outside the battery to power our circuit.
Anode Oxidation
This first part of the redox reaction, oxidation, occurs between the anode and electrolyte, and it produces electrons(marked as e-).
Some oxidation reactions produce ions, such as in a lithium-ion battery. In other chemistries, the reaction consumes ions, like in the common alkaline battery. In either case, ions are able to flow freely through the electrolyte where electrons cannot.
Cathode Reduction
The other half of the redox reaction, reduction, occurs in or near the cathode. Electrons produced by the oxidation reaction are consumed during reduction.
In some cases, like lithium-ion batteries, positively charged lithium ions produced during the oxidation reaction are consumed during reduction. In other cases, like alkaline batteries, negatively charged ions are produced during reduction.
Electron Flow
In most batteries, some or all of the chemical reactions can occur even when the battery is not connected to a circuit. These reactions can impact a battery’s shelf life.
For the most part, the reactions will only occur at full force when an electrically conductive circuit is completed between the anode and cathode. The less resistance between the anode and cathode, the more electrons are allowed to flow, and the quicker the chemical reactions occur.

Creating a short circuit in a battery (even accidental ones, in this case), can be dangerous. Lithium-ion batteries are known to overheat and even smoke or catch fire in the presence of a short circuit.
We can pass these moving electrons through various electrical components, known as a “load,” in order to accomplish something useful. In the motion graphic at the beginning of this section, we are lighting a virtual light bulb with our moving electrons.
Dead Battery
The chemicals in the battery will ultimately reach a state of equilibrium. In this state, the chemicals will no longer have a tendency to react, and as a result, the battery will not generate any more electric current. At this point, the battery is considered “dead.”
Primary cells must be disposed when the battery is dead. Secondary cells can be recharged, and this is accomplished by applying a reverse electric current through the battery. Recharging occurs when the chemicals perform another series of reactions to take them back to their original state.
Terminology
People often use a common set of terms when talking about a battery’s voltage, capacity, current sourcing capability and so on.
Cell
A cell refers to a single anode and cathode separated by electrolyte used to produce a voltage and current. A battery can be made up of one or more cells. A single AA battery, for example, is one cell. Car batteries contain six cells at 2.1 V each.

The common 9-volt battery contains six 1.5 V alkaline cells stacked on top of each other
Primary
Primary cells contain chemistry that cannot be reversed. As a result, the battery must be thrown away after it is dead.
Secondary
Secondary cells can be recharged and have their chemistry reverted back to their original state. Otherwise known as “rechargeable batteries,” these cells can be used many times.
Nominal Voltage
The nominal voltage of a battery is the voltage stated by the manufacturer.
For example, alkaline AA batteries are listed as having 1.5 V. This article from Mad Scientist Hut shows their tested alkaline batteries start at about 1.55 V and then slowly lose voltage as they are discharged. In this example, “1.5 V” nominal voltage refers to the maximum or starting voltage of the battery.
This Storm battery pack for quadcopters shows the discharge curve for their LiPo cells starting at around 4.2 V and dropping to around 2.8 V as it discharges. The nominal voltage listed for most lithium-ion and LiPo cells is 3.7 V. In this case, “3.7 V” nominal voltage refers to the average voltage of the battery over its discharge cycle.
Capacity
A battery’s capacity is a measure of the amount of electric charge it can deliver at a specific voltage. Most batteries are rated in amp hours (Ah) or milliamp hours (mAh).
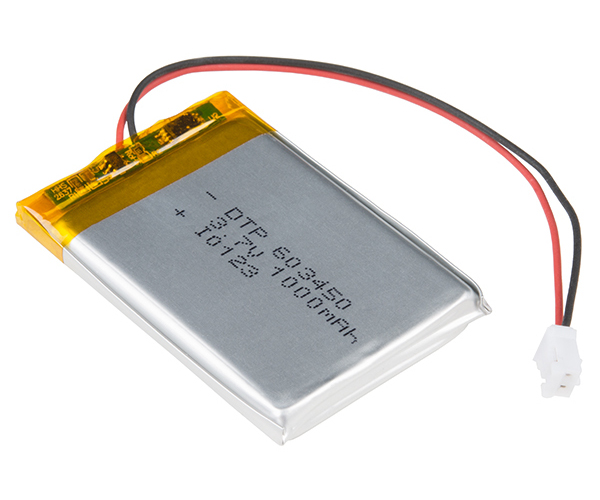
This LiPo battery is rated for 1000 mAh, which means it can provide 1 amp for 1 hour before it is considered dead.
Most battery discharge graphs show the battery’s voltage as a function of capacity, such as these AA battery tests by PowerStream. To figure out if a battery has enough capacity to power your circuit, find the lowest acceptable voltage and find the associated mAh or Ah rating.
C-Rate
Many batteries, especially powerful lithium-ion batteries, express discharge current as “C-Rate” in order to more clearly define battery attributes. C-Rate is the rate of discharge relative to the battery’s maximum capacity.
1C is the amount of current required to discharge the battery in 1 hour. For example, a 400 mAh battery supplying 1C of current would be supplying 400 mA. 5C for the same battery would be 2 A.
Most batteries lose capacity at higher current draws. For example, this product info graph from Chargery shows that their LiPo cell has less mAh at higher C-Rates.
NOTE: General advice holds that you should charge LiPo batteries at 1C or less.
Usage
Single Cell
Some circuits can be powered by a single cell, but make sure that the battery can provide enough voltage and current.
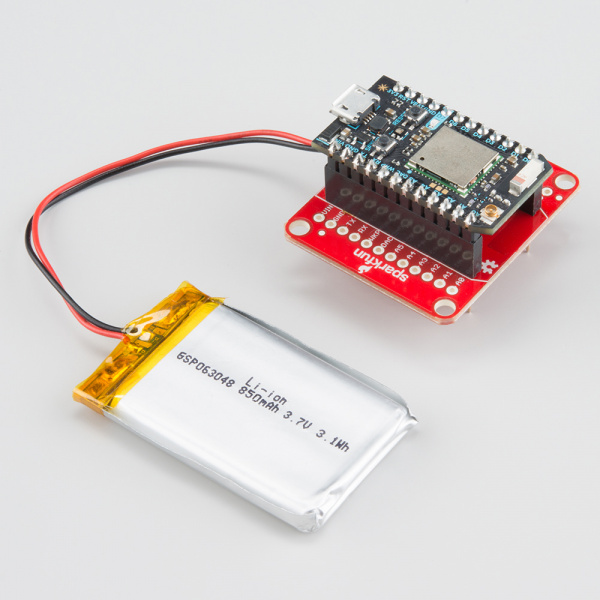
This Photon Battery Shield is being powered from a single LiPo cell
If the voltage is too high or too low for your circuit, you will likely need a DC/DC converter.
Series
In order to increase the voltage between a battery’s terminals, you can place the cells in series. Series means stacking the cells end-to-end, connecting the anode of one to the cathode of the next.
By connecting batteries in series, you increase the total voltage. Add the voltage of all the cells to determine the operating voltage. The capacity stays the same.
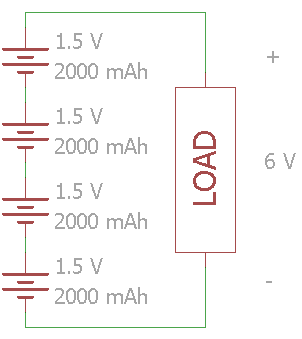
In this example, four 1.5 V cells are connected in series. The voltage across the load is 6 V while the total set of batteries have a 2000 mAh capacity.
In most consumer electronics that use alkaline batteries, the batteries are stacked in series. For example, this 2x AA battery holder can raise the nominal voltage to 3 V for a project.
NOTE: If you are charging lithium-ion or LiPo batteries in series, you need to make sure to use special circuitry known as a "balancer" to ensure the voltages among the cells stays even. Some chargers, like this one, have balancers to allow for safe charging.
Parallel
If the voltage of a single cell is adequate for the load, you can add batteries in parallel to increase the capacity. Note that this also means increasing the available current (C-Rate).
Be careful when connecting batteries in parallel! All the cells should have the same nominal voltage and same charge level. If there are any voltage differences, a short circuit could occur causing overheating and possibly fire.
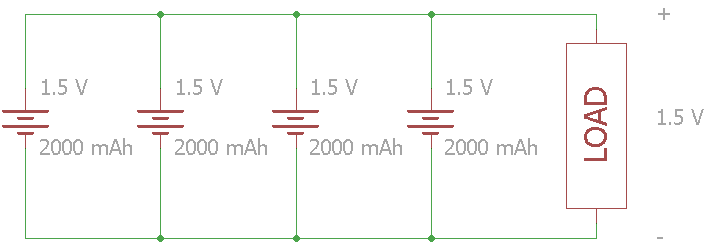
In this example, four 1.5 V cells are connected in parallel. The voltage across the load stays at 1.5 V, but the total capacity increases to 8000 mAh.
Series and Parallel
If you want to increase voltage and capacity, you can combine series and parallel batteries. Once again, make sure that the voltage level is the same for the batteries in parallel, as a short circuit can occur.
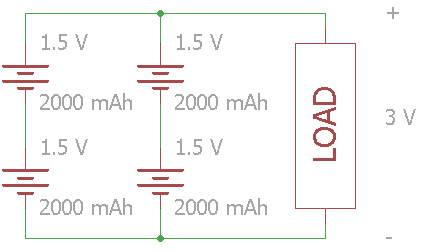
In this example, the total voltage across the load is 3V, and the batteries' combined capacity is 4000 mAh.
In large battery packs, especially lithium-ion, you often see the configuration listed using ’S' and ‘P’ for series and parallel. The configuration for the circuit above is 2S2P. As a practical example, modern electric cars use massive arrays of batteries connected in series and parallel.
Resources and Going Further
By now, you should have an understanding of how batteries were invented and how they work. Batteries are one method of providing electric energy to your project, and they can be incredibly useful if you need a portable power source.